Identifying the charge state of individual adsorbates
EPR-STM was demonstrated to achieve single spin sensitivity and sub-nanometer spatial resolution and has been used to gain insight into the interaction of the nuclear and electronic magnetic moments of single atoms, the dipolar and exchange coupling between atoms and molecules, and the coherent spin dynamics of atoms and molecules on a surface. So far, all of the recent magnetoresistive EPR-STM studies were performed on 3d transition-metal atoms, i.e., Fe, Ti, and Cu or an Fe ion in the iron phthalocyanine molecule all adsorbed on the MgO surface. The application of this technique to other elemental systems and to a broader range of problems beyond single-atom magnetism remains to be demonstrated.
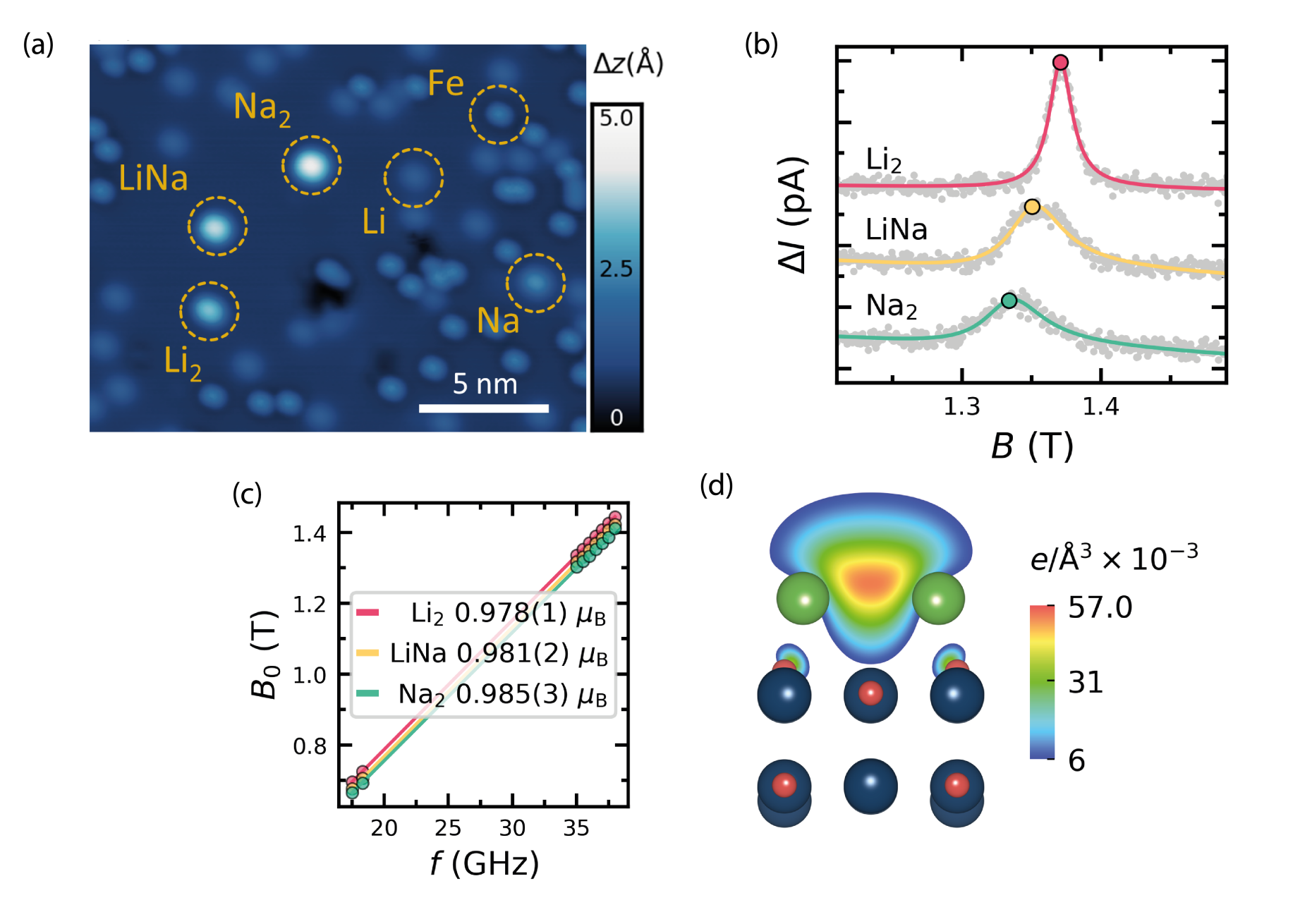
In our work we show that EPR-STM can be applied to study the electronic configuration of alkali electron donor complexes on an oxide surface [1]. Functionalized oxide surfaces are of particular importance for heterogeneous catalysis. A widely used system for fundamental studies in heterogeneous catalysis is MgO(100) with a small amount of metal atoms or clusters added to the bulk or surface. The doping of MgO with alkali metals transforms the bare MgO substrate into a catalyst suitable for industry-relevant reactions, e.g., oxidative coupling of methane to produce higher order hydrocarbons. In such systems, the chemical transformation is thought to occur through the formation of electron-rich oxygen sites in the vicinity of the alkali atoms. However, the electronic state and chemical bonds of the dopant atoms, which are crucial for the catalytic activity, can only be determined by means of density functional theory (DFT). Here, we use EPR-STM to investigate the charge state and binding configuration of individual Li and Na atoms as well as their dimers (Li2, Na2, and LiNa) deposited on ultrathin MgO(001) layers grown on a Ag substrate, see Figure. Our measurements reveal a distinct EPR signal corresponding to a magnetic moment of 1 µB for paramagnetic alkali metal dimers adsorbed near the top oxygen sites of MgO. In contrast, single alkali atoms adsorb on bridge O sites and show no magnetic moment. The absence of an EPR signal in the alkali atoms indicates a change of the orbital occupancy relative to the gas phase, where the valence s-shell is singly occupied. This change in occupancy shows that the atoms and dimers have the same charge state +1e and that both transfer exactly one electron to the Ag substrate.
Our work sheds light on the bonding and charge state of alkali metal adsorbates on ultrathin MgO/Ag(001) and opens a new range of systems for EPR-STM investigations beyond 3d transition metals. The ability to probe electron donor species using EPR-STM may be useful to perform atomic-scale characterization of silicon-based spin qubits and deterministic manipulation of dopants in semiconductors and molecular adsorbates. Other systems of interests are alkali metal doped graphene and organic semiconductors, where charge transfer from the different alkali metal species induces a superconducting phase transition.
Reference
[1] S. Kovarik, R. Robles, R. Schlitz, T. S. Seifert, N. Lorente, P. Gambardella, and S. Stepanow, Electron Paramagnetic Resonance of Alkali Metal Atoms and Dimers on Ultrathin MgO, Nano Lett. 22, 10, 4176–4181 (2022) Download article (PDF, 2.3 MB)