Atom-by-atom doping of single molecules: a route to charge and spin control in organic electronics
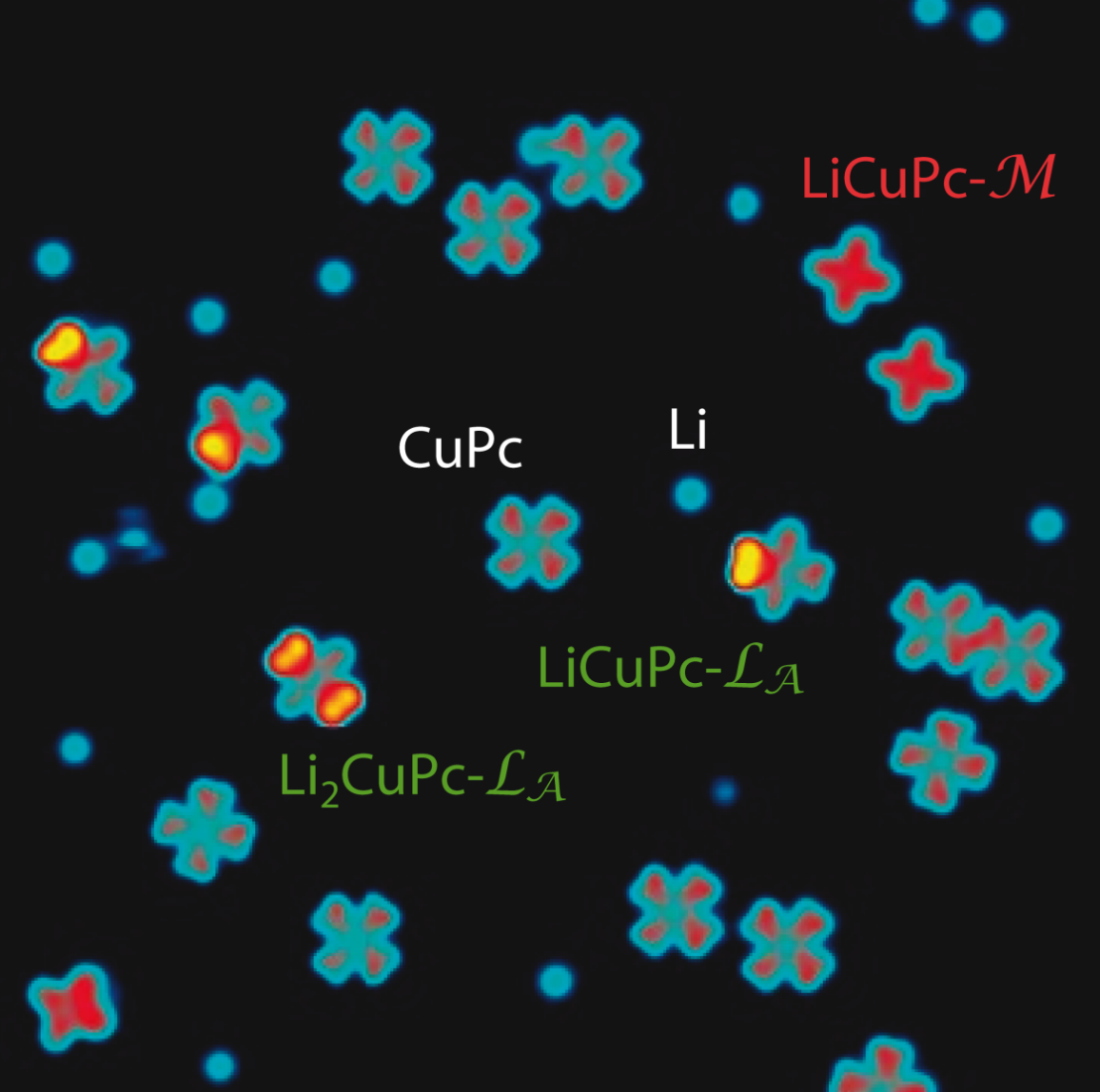
Similar to silicon-based electronics, chemical doping offers a tool for tailoring the electrical characteristics of organic semiconductors. However, unlike for inorganic materials used in electronics applications, controlling the influence of dopants in molecular complexes is complicated by the presence of multiple doping sites, electron acceptor levels, and intramolecular correlation effects. Our team has used the tip of a scanning tunneling microscope to control the position of dopant atoms within a single molecule and probe their electrical and magnetic properties with sub-nanometer spatial resolution. We showed that phthalocyanine molecules, a model system used in the fabrication of organic field effect transistors, light emitting devices, and photovoltaic cells, can host at least three distinct doping sites and up to six dopant atoms. Ligand or metal orbitals can be selectively charged by modifying the position of the dopants inside the molecule, whereas the magnitude of the conductance gap is found to be sensitive to the molecule-dopant separation. Finally, owing to strong spin-charge correlation in confined molecular orbitals, we showed that charge donation by alkali atoms provides an effective way to tune the molecular spin without resorting to magnetic dopants. The detection of single dopant effects in individual molecules and the ability to manipulate such dopants may be envisioned as a first step towards the realization of molecular devices with magnetic and optoelectronic properties governed by ultimate discretization effects.
Selected publications
- Site and orbital-dependent charge donation and spin manipulation in electron doped metal-phthalocyanines, C. Krull, R. Robles, A. Mugarza, and P. Gambardella, Nature Mater. 12, 337 (2013). Download article (PDF, 1.8 MB)
- Spin tuning of electron-doped metal-phthalocyanine layers, S. Stepanow, A. Lodi Rizzini, C. Krull, J. Kavich, J. C. Cezar, F. Yakhou-Harris, P. M. Sheverdyaeva, P. Moras, C. Carbone, G. Ceballos, A. Mugarza, P. Gambardella, J. Am. Chem. Soc. 136, 5451 (2014). Download article (PDF, 3 MB) Download supplementary information (PDF, 308 KB)