Tuning the spin of 3d-metal phthalocyanines by alkali metal electron donors
Thin phthalocyanine films have been incorporated in various devices including field effect transistors, light-emitting devices and photovoltaic cells. Phthalocyanines can accommodate a variety of metal ions in their center that have particular spin configurations rendering this class of compounds also interesting for magnetic materials. Due to the particular coordination geometry of the metal ions the spin states of the complexes are sensitive to their local chemical environment. Contacting the molecules to metal electrodes can therefore largely influence the magnetic properties of the metal centers. On the other hand, direct chemical doping offers an effective route for tailoring the electronic and magnetic characteristics of molecular magnets.
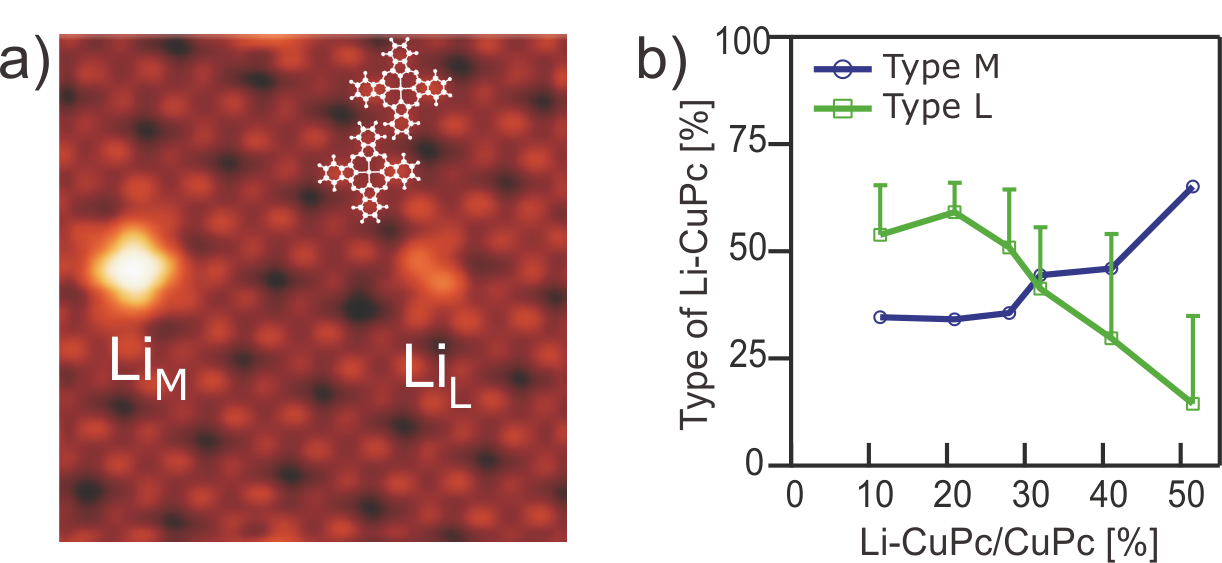
The charging sequence of metal-phthalocyanines involves both ligand and metal orbitals and depends strongly on the nature of the central metal ion. Doping of phthalocyanine chemisorbed on a metal substrate is further complicated due to the interplay of additional substrate induced charging and screening effects that can alter the role of the charge donor towards the molecules. The step-wise attachment of electron donor Li atoms to chemisorbed CuPc and NiPc molecules was recently investigated by scanning probe microscopy and DFT calculations. The effects of alkali metal doping on the magnetic properties of metal-phthalocyanines, however, have not been studied in a systematic way, neither in the bulk nor at interfaces, which is of importance for molecular electronic and spintronic applications.
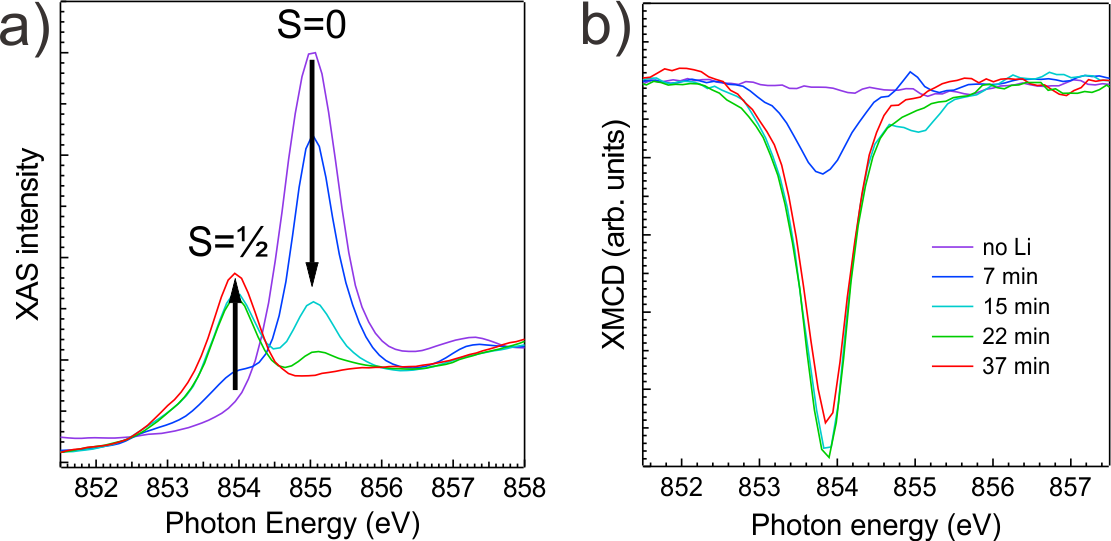
Figure 2 shows the x-ray absorption and XMCD spectra of NiPc as a function of Li doping. With increasing Li concentration the pristine non-magnetic Ni ions, which have a spin singlet d8 configuration, are reduced and assume a d9 configuration (Figure 2a). At the same time a magnetic moment corresponding to S=1/2 appears, as evidenced by the XMCD (Figure 2b). For CuPc the Li doping has the opposite effect; the magnetic moment can be turned off by Li doping, due to the complete filling of the d-shell (from d9 to d10). In MnPc, on the other hand, we observe no changes of the d5 metal ion valence state as a function of doping, but a transition from an intermediate S=3/2 to a high S=5/2 spin state. This transition is due to a strong reduction of the ligand field induced by Li, which is also observed for CuPc, NiPc, and FePc.
When combined with STM studies (Figure 1), these results provide a complete picture of chemically doped metal-phthalocyanines on surfaces, in which strong correlation effects induce site-specific charging and magnetic effects. Moreover, these results demonstrate that different spin states can be realized in metal-phthalocyanine layers interfaced with metals by doping with Li atoms, offering a route to tune the magnetic properties of surface-supported molecular systems employing non-magnetic dopants.
Selected publications
- Spin tuning of electron-doped metal-phthalocyanine layers, S. Stepanow, A. Lodi Rizzini, C. Krull, J. Kavich, J. C. Cezar, F. Yakhou-Harris, P. M. Sheverdyaeva, P. Moras, C. Carbone, G. Ceballos, A. Mugarza, P. Gambardella, J. Am. Chem. Soc. 136, 5451 (2014). Download article (PDF, 2.4 MB) Download supplementary information (PDF, 308 KB)